
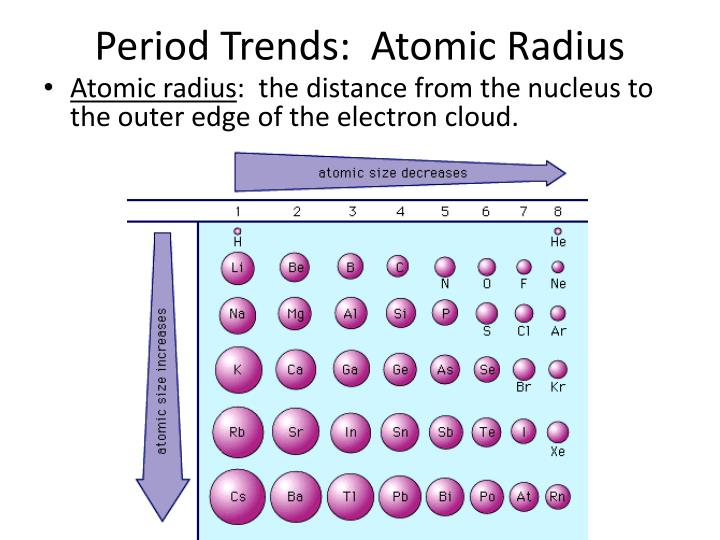
They are pulled in closer to the nucleus and the atom gets smaller. If the valence electrons feel a greater attraction Since valenceĮlectrons are all in the same level, they feel a greater attraction to the nucleusĪs we move across the period. Each time we add a proton to a the nucleus the electron (whichĪre negatively charged) feel a greater attraction to the nucleus. Protons in the nucleus, is also increasing. RememberĪlso that as we move from sodium to argon, the nuclear charge, the number of In the outer most level of the atom (the furthest from the nucleus). Remember, and this is important, the valence electrons are Sodium has one valence electron and as we move across to argon with eight To get started we must consider the electron configuration for the eight elements Several new ideas valence electrons, inner core electrons, effective nuclearĬharge and shielding. To develop an explanation of the trends in atomic radii we need to discuss Yet it is one of the largest atoms, not the smallest. That mightĮxplain the trend in atomic radii going across a period but it does notĮxplain the trend going down a group. We might expect that the more protons the smaller the radius. So we could argue that going from hydrogen through the elements the Going across a period the radius decreases. The trend within a group, but when we look at the atomic radius trend We might be able to use that argument to explain The like charges, and the size of the atom will increase. How do we understand these two trends? Explanations of trends in atomic radii that do not work perfectly: We might argue that going from hydrogen through the elements that the Also notice that going across a period the atomic Looking at the table above notice from lithium to cesium, going down the group, Radii for metals can be estimated from the distance between metal (we'll discuss the term covalent in the next chapter) Atomic After collecting large amounts of data generally acceptedĪtomic radii are known for most elements in the periodic table.Ītomic radii determine this way are also called covalent radii. Taken in these types of determinations however. Additional distances canīe obtained from other distances between atoms. Therefore theĪtomic radius of chlorine is 0.994 Å. Nuclei is the sum of two chlorine atomic radii. To get the atomic radius we assume the distance between the two The two chlorine atoms in Cl 2 is known to be 1.988 Å. It is difficult toĭefine a sharp boundary for distance between the electrons in any Very broad region for finding the electron.
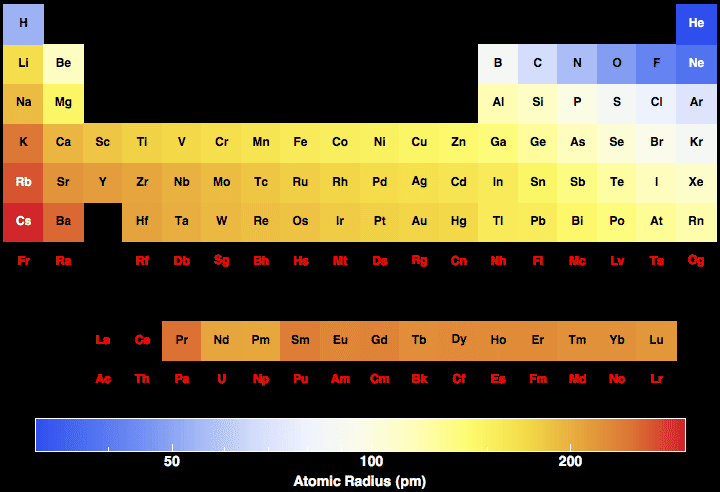
Our quantum mechanical description of an atom suggests a The immediate question is what is an atomic The first property to explore isĪtomic radius. The Quantum Mechanical model of theĪtom can 'tested' by looking at the experimental data of atomic The strength of any model is in it ability to explainĮxperimental observations. The first property to explore is atomic radius.


 0 kommentar(er)
0 kommentar(er)
